GALVANIZING
What is Hot Dip Galvanizing?
Galvanized coatings are applied to iron and steel primarily to provide protection against corrosion of the basis metal. Hot Dip Galvanizing is a process in which an adherent, protective coating of zinc and zinc compounds is developed on the surfaces of iron and steel products by immersing them in a bath of molten zinc. The protective coatings usually consist of several layers. Those closest to the basis metal are composed of iron-zinc compounds these in turn are covered by an outer layer consisting almost entirely of zinc. The complex structure of layers that comprise a galvanized coating varies greatly in chemical composition and physical and mechanical properties, being affected by chemical activity, diffusion, and subsequent cooling. Small differences in coating composition, bath temperature, time of immersion, and rate of cooling or subsequent reheating can result in significant changes in the appearance and properties of the coating.
Where is it used?
Galvanized steel is widely used in applications where corrosion resistance is needed.
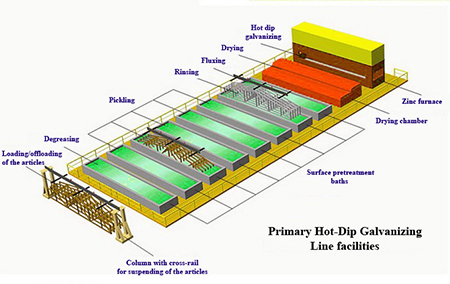
Process
Surface Preparation / Pre-Treatment
The galvanizing reaction will only occur on a chemically clean surface.
Like most coating processes the secret to achieving a good quality coating lies in the preparation of the surface
Degreasing
It is essential that the article is free of grease, dirt and scale before galvanizing.
Contamination is removed by a series of processes. Firstly we degrease using a caustic solution into which the component is dipped.
Pickling
The article is then rinsed and then dipped in
hydrochloric acid at ambient temperature to remove rust and mill scale<
Rinsing
The article is then rinsed in pure water to remove
all the acid content from the surface of the articles.
Fluxing
The article is then dipped in Zinc ammonium chloride solution
otherwise called as the flux solution at a temp of 65 – 75 deg C
Galvanizing / Dipping
The clean iron or steel component is dipped into the molten zinc (at 450°C),
a series of zinc-iron alloy layers are formed by a metallurgical reaction between the iron and zinc.
Post-Treatments
Quenching
The galvanized components are quenched in water to reduce the temperature after galvanizing
Dichromating
After quenching the materials are dipped in the di-chromate solution, which acts as a strong reducing agent
and prevents further oxidation of the pure zinc layer to form Zinc hydroxide or White rust.